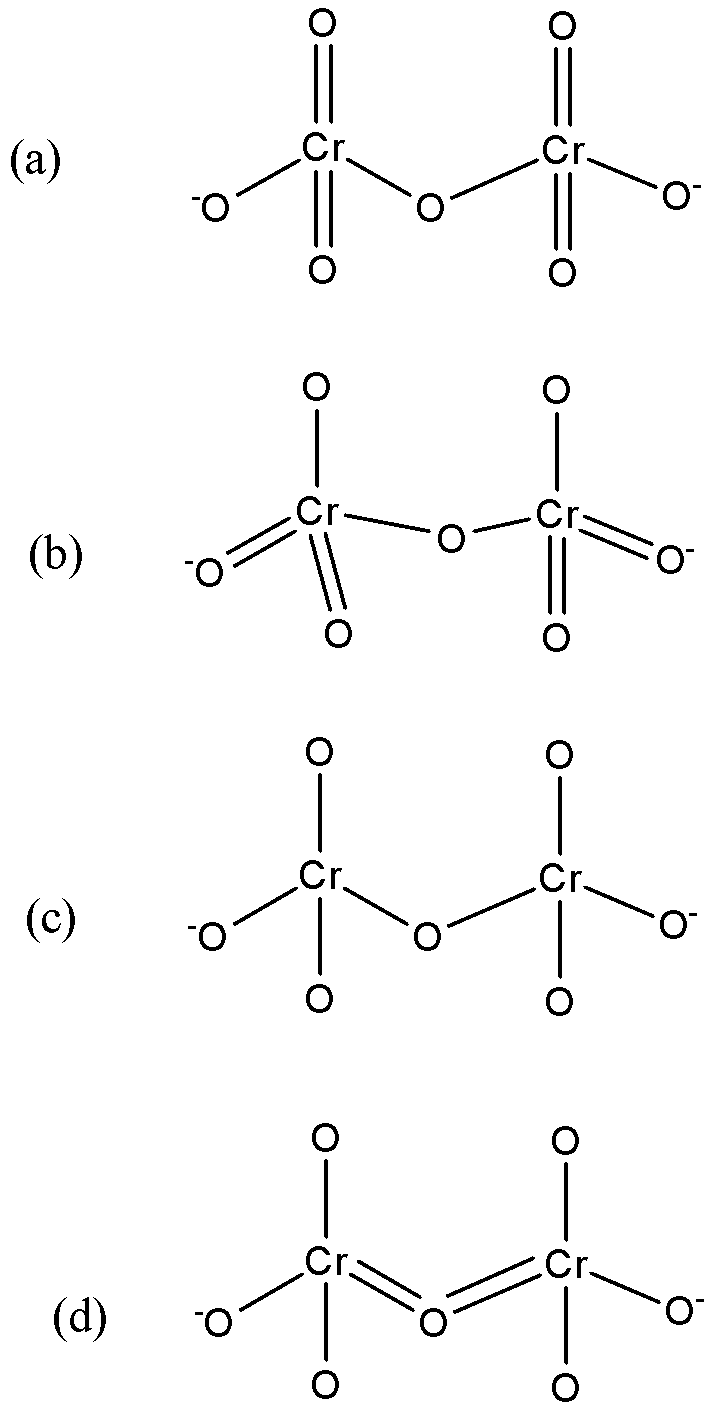
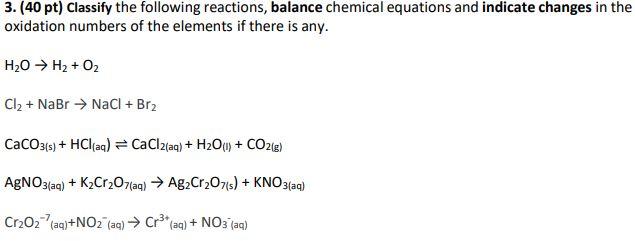Another Look at Photoelectron Spectra of the Anion Cr2O2–: Multireference Character and Energetic Degeneracy | Journal of Chemical Theory and Computation

Balance the following reaction by oxidation number method: K2Cr2O7 + FeSO4 + H2SO4→ K2SO4 + Cr2(SO4)3 + Fe2(SO4)3 + H2O .

Another Look at Photoelectron Spectra of the Anion Cr2O2–: Multireference Character and Energetic Degeneracy | Journal of Chemical Theory and Computation

Cu(s) + NO 3^- (aq) + H ^ + (aq) → Cu ^2 + (aq) + NO 2 (g) + H 2 O(l)If the above equation is balanced with lowest whole number coefficients, find the coefficient for H + (aq).

Balance the following redox reactions by ion electron method. Cr2O^2 - 7 + SO2(g)→ Cr^3 + (aq) + SO^2 - 4(aq) (in acidic solution)

Visible-Light-Promoted Photocatalytic Applications of Carbon Dots: A Review | ACS Applied Nano Materials